Answer: -
10.5% is the Weight / weight % or percent by mass of sodium sulfate
Explanation: -
Mass of sodium sulfate = 11.2 g
Mass of water = 95.0 g
Total mass of the solution = Mass of sodium sulfate + Mass of water
= 11.2 + 95.0 = 106.2 g
Weight / weight % or percent by mass of the solute = (Mass of sodium sulfate / Total mass of the solution) x 100
=
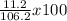
= 10.5 correct to 3 significant figures.