Answer: Option (A) is the correct answer.
Step-by-step explanation:
When a neutral atom loses an electron then it acquires a negative charge and if a neutral atom gains an electron then it acquires a positive charge. Hence, an ion is formed in both the cases.
For example, sodium on losing an electron become
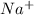 ion.
ion.
An isotope is defined as the specie which contains same number of protons but different number of neutrons.
For example,
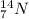 and
and
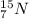 are isotopes.
are isotopes.
A molecule is defined as a substance that contains chemically combined two or more number of atoms of an element.
For example,
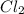 is a molecule.
is a molecule.
Whereas a compound is defined as a substance in which different atoms of different elements chemically combine together in a fixed ratio by mass.
For example,
 is a compound.
is a compound.
Thus, we can conclude that an ion is an electrically charged atom.