Answer: lithium fluoride
Explanation: Lithium atom has oxidation number of +1.
Electronic configuration of lithium:
![[Li]:3:1s^22s^1](https://img.qammunity.org/2019/formulas/physics/middle-school/kve4cndu7hld5a77rpppsr23afcpg15vbj.png)
Lithium atom will loose one electron to gain noble gas configuration and form lithium cation with +1 charge.
![[Li^+]:2:1s^2](https://img.qammunity.org/2019/formulas/physics/middle-school/yje712pcy0h1iifuudadn3bqfuqdj797x7.png)
Fluorine atom has oxidation number of -1.
Electronic configuration of fluorine:
![[F]:9:1s^22s^22p^5](https://img.qammunity.org/2019/formulas/physics/middle-school/bdhbqni7qu28e7hog27qovtrc9iwdtk7b0.png)
Flourine atom will gain one electron to gain noble gas configuration and form fluoride ion with -1 charge.
![[F^-]:10:1s^22s^22p^63s^23p^6](https://img.qammunity.org/2019/formulas/physics/middle-school/ifh6ic0cy9xms7k68mhbgtfctjlud3b7x8.png)
In lithium fluoride the one electron from lithium metal gets transferred to fluorine atom to form
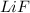 .
.
The nomenclature of ionic compounds is given by:
1. Positive ion is written first.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
Hence, the name of
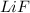 is lithium fluoride.
is lithium fluoride.