The question is incomplete.
The complete question should be
How many grams of sodium can react with 750 mL of a 6.0 M solution of sulfuric acid, H₂SO₄?
Answer: -
Strength of H₂SO₄ = 6.0 M
Volume of H₂SO₄ = 750 mL = 750/1000 = 0.75 L
Number of moles of H₂SO₄ = 0.75 L x 6.0 M
= 4.5 mole of H₂SO₄
The balanced chemical equation for this reaction is
2 Na + H₂SO₄ → Na₂SO₄ + H₂
From the balanced chemical equation we see
1 mole of H₂SO₄ react with 2 mole of Na
4.5 mole of H₂SO₄ react with
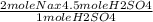 mole of Na
mole of Na
= 9 mole of Na
Atomic mass of Na = 23 g/mol
Mass of Na = 9 mol x 23 g/ mol
= 207 g
Thus 207 grams of sodium can react with 750 mL of a 6.0 M solution of sulfuric acid, H₂SO₄