Hello!
Data:
Molar Mass of H2CO3 (carbonic acid)
H = 2*1 = 2 amu
C = 1*12 = 12 amu
O = 3*16 = 48 amu
------------------------
Molar Mass of H2CO3 = 2 + 12 + 48 = 62 g/mol
Now, since the Molarity and ionization constant has been supplied, we will find the degree of ionization, let us see:
M (molarity) = 0.01 M (Mol/L) →
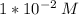
Use: Ka (ionization constant) =
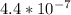
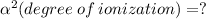
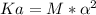
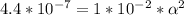
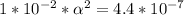
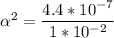
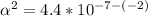
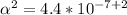
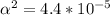
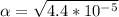
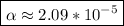
Now, we will calculate the amount of Hydronium [H3O+] in carbonic acid (H2CO3), multiply the acid molarity by the degree of ionization, we will have:
![[ H_(3) O^+] = M* \alpha](https://img.qammunity.org/2019/formulas/chemistry/middle-school/akf88upwqbcty3c7jqv322xc0vctc1cwfj.png)
![[ H_(3) O^+] = 1*10^(-2)* 2.09*10^(-5)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/raql3spwhzau9jnmfhvtudibfiwvhikx8x.png)
![[ H_(3) O^+] = 2.09*10^(-2-5)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/h93ime5s1f9ku2ukq2qx4cvb17gj8lmxkh.png)
![\boxed{[ H_(3) O^+] = 2.09*10^(-7)}](https://img.qammunity.org/2019/formulas/chemistry/middle-school/cgyomkdm5h3uezhex1po52hghj7y475hen.png)
And finally, we will use the data found and put in the logarithmic equation of the PH, thus:
Data:
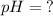
![[ H_(3) O^+] = 2.09*10^(-7)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bo2cv8ocw8g87dpslyq6x6zrowm9v35er6.png)
apply the data to formula
![pH = - log[H_(3) O^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/rwjqkzcaszmdmqaa3z503a15075auonv43.png)
![pH = - log[2.09*10^(-7)]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/nnayx80k7eharvskvdzpt8rbvuycvo54bg.png)

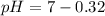
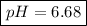
Note:. The pH <7, then we have an acidic solution (weak acid).
Now, let's find pOH by the following formula:
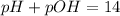
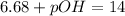
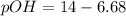

I Hope this helps, greetings ... DexteR! =)