Answer: -
2.5 mL
Explanation: -
Volume of final solution = 10 mL
Strength of final solution = 10 mg / mL
Amount of drug required = Volume of final solution x strength of final solution
= 10 mL x 10 mg/ mL
= 100 mg.
Strength of supplied drug = 40mg/ mL
Volume of supplied drug required =
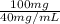
= 2.5 mL
Thus to make 10 ml of a 10 mg/ml solution. 2.5 mL will be needed.