Information from part a is missing in the question which is given below.
Part a ) The titration of 25.0 mL of an iron(II) solution required 18.0 mL of a 0.225 M solution of dichromate to reach the equivalence point. What is the molarity of the iron(II) solution?
Balanced chemical equation for the reaction is

Let us use the information from part a to find grams of Fe.
Step 1 : Find moles of dichromate solution
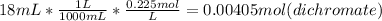
Step 2 : Find moles of Fe(II) solution using balanced equation and mol dichromate
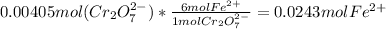
Step 3 : Convert moles of Fe(II) to grams of Fe
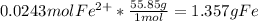
Step 4 : Find % of Fe by mass
Grams of Fe present in the ore are 1.357
Grams of ore are given as 4.05
% of Fe in the ore can be found out using following formula
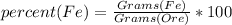
% Fe by mass =
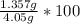
% Fe by mass = 33.5%
Percentage of Fe by mass in the ore is 33.5%