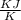Given information :
G = 173.3 KJ
H = 180.7 KJ
T = 303.0 K
S = unknown (?)
By using the given formula : G = H - TS , we can calculate the value of 'S'
On rearranging the formula we get : S =
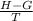
Plug in the value of G , H and T in the above formula :
S =
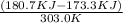
S = 0.02442