Answer:- 55 g of LiCl are required to make 2.0 L of 0.65 M solution.
Solution:- Given-
molarity of the solution = 0.65M
Volume of solution = 2.0 L
molarity of solution =
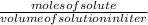
It could be arranged as...
moles of solute = molarity x volume of solution
moles of solute = 0.65 x 2.0 = 1.3 moles
Molar mass of LiCl is 42.39 g/mol.
So, mass of LiCl required = 1.3 mol x 42.39g/mol = 55 g