M = mass of aluminium = 1.11 kg
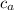 = specific heat of aluminium = 900
= specific heat of aluminium = 900
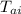 = initial temperature of aluminium = 78.3 c
= initial temperature of aluminium = 78.3 c
m = mass of water = 0.210 kg
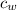 = specific heat of water = 4186
= specific heat of water = 4186
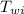 = initial temperature of water = 15 c
= initial temperature of water = 15 c
T = final equilibrium temperature = ?
using conservation of heat
Heat lost by aluminium = heat gained by water
M
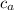 (
(
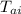 - T) = m
- T) = m
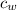 (T -
(T -
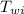 )
)
(1.11) (900) (78.3 - T) = (0.210) (4186) (T - 15)
T = 48.7 c