A precipitation reaction is a chemical reaction in which two soluble salts (ionic compounds) in an aqueous solution (solution with water as the solvent) yield insoluble, solid salt, a precipitate, as one of the products. It is generally a double replacement reaction, in which there are two ionic compounds, and their ions switch (AB+CD yield AC+BD).
Example:
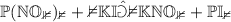
N.B. The (s) indicates a solid (or precipitate), whereas (aq) means that the compound is dissolved in an aqueous (water-containing) solution.