I'm not sure the list of the compounds you have, but
carbon tetrachloride,
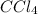 , will dissolve compounds that are nonpolar
, will dissolve compounds that are nonpolar.
'Like dissolves like' is a general saying that is good to use. So if you know the polarity of the compound, you can determine many different types of compounds that would dissolve in it.
Carbon tetrachloride is therefore nonpolar. It is nonpolar due to the presence of a dipole-dipole moment, in which all 4 of the chlorides have an equal 'pull' on the carbon.