When comparing concentration and volume, we can use the equation:
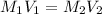
**Note: the volume in this equation is in liters. Be sure to convert mL to L by dividing by 1000.
Now let's plug in the values that were given to us in the equation (using 0.050L for 50 mL and 0.200L for 200 mL):
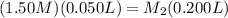
Then we can solve for
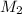
:
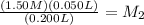
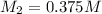
So now we know that
the resulting solution has a molarity of 0.375 M.