Answer 1) Option A) 58.05
In the given reaction of iron forming rust when reacts with the oxygen.
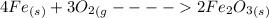
We can clearly see that, 4 moles of iron reacts with 3 moles of oxygen to give 2 moles of iron oxide.
So 4 Fe : 3 O and 77.4 moles of Fe : x moles of O
(3 X 77.4) / 4 = 58.05
So when we solve we get x as 58.05.
Hence the no. of moles of oxygen will be 58.
Answer 2) Option A) 10.03
The number of moles of carbon dioxide produced when 161.0 g of methane undergoes combustion will be 10.03
as we know the molar mass of methane is 16.043 g
As we can see in the reaction the mole ratio is 1:1;
1 mole of methane produces 1 mole of carbondioxide.
So, 161 g / 16.043 g = 10.03 moles of Carbon dioxide.