Answer: Option (C) is the correct answer.
Step-by-step explanation:
At high temperature, molecules will have more kinetic energy and therefore, they will rapidly move from one place to another.
Also, kinetic energy is directly proportional to temperature. That is, more is the temperature more will be kinetic energy of molecules of a substance.
Whereas less is the temperature less will be kinetic energy of molecules of a substance.
Therefore, when temperature changes from
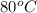 to
to
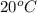 then kinetic energy will decrease.
then kinetic energy will decrease.
Hence, we can conclude that the statement kinetic energy decreases as temperature decreases is happening in this system.