Answer:
The strong base is with
 concentration equals to
concentration equals to
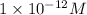 .
.
Step-by-step explanation:
The pH of the solution is defined as negative logarithm of
 ions in a solution.Higher the
ions in a solution.Higher the
 ions concentration lower will be the value of pH and more acidic will be the solution.
ions concentration lower will be the value of pH and more acidic will be the solution.
![pH=-\log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/vwilut25e4cux34589pwoorivy6w6y51xe.png)
1)
![[H+] = 1 * 10^(-9) M](https://img.qammunity.org/2019/formulas/chemistry/high-school/eqyr0mnj9qx4jr69dv7od8ra5jb4qfve3d.png)
![pH=-\log[1 * 10^(-9) M]=9](https://img.qammunity.org/2019/formulas/chemistry/high-school/c51s8j99ewvp2z802lt3x5uwltvieyw72a.png) Most acidic solution
Most acidic solution
2)
![[H+] = 1 * 10^(-11) M](https://img.qammunity.org/2019/formulas/chemistry/high-school/mx8plqwgkxripcvesf20szdbt8mawhmp7q.png)
![pH=-\log[1 * 10^(11) M]=11](https://img.qammunity.org/2019/formulas/chemistry/high-school/3ihtz2oqu1oqkyk47fsc91ksgpaot9np3k.png)
3)
![[H+] = 1 * 10^(-10) M](https://img.qammunity.org/2019/formulas/chemistry/high-school/vhtqlp8c844iep8ld1c0kpa5f4d233amec.png)
![pH=-\log[1 * 10^(-10) M]=10](https://img.qammunity.org/2019/formulas/chemistry/high-school/3k9r2zi1krhmyttmi7mpp2x86sauklfh6f.png)
4)
![[H+] = 1 * 10^(-12) M](https://img.qammunity.org/2019/formulas/chemistry/high-school/y2x1462szzwb383x5unnmc3g9tphxhu4lv.png)
![pH=-\log[1 * 10^(-12) M]=12](https://img.qammunity.org/2019/formulas/chemistry/high-school/p8k8szzoifqeygtdhtiz5fqh9iqgn9czmz.png) Most basic solution
Most basic solution