Hey there!
The formula for density is d = m/v.
The mass of the sample is 56.5 g, and the volume of the sample is 5 cm³.
So, to plug these values into the equation, we have:
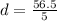
Simplified, this is:
d = 11.3
The unit for this is g/cm³, or grams per cubic centimeter.
So, the density of lead is 11.3 g/cm³.
Hope this helps!