Answer :
(1) The missing component is
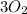 .
.
(2) The missing component is
 .
.
Explanation :
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
Part 1 :
Decomposition reaction : It defined as the reaction in which larger reactant molecules decomposes to give two or more smaller products molecule.
The balanced chemical reaction will be:
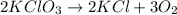
Thus, in this reaction the missing component is
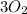 .
.
Part 2 :
Synthesis reaction : It is defined as the chemical reaction where multiple substances or reactants combine to form a single product.
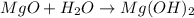
Thus, in this chemical reaction the missing component is
 .
.