Answer : The pH of calcium acetate solution will be 9.38.
Explanation : When calcium acetate dissocates completely in solution to form Calcium(+2) ions and two acetate ions, this gives the acetate ion concentration as (2 X 0.51 M) = 1.02 M
Acetate ion then hydrolyzes as follows;
 :
:
Now, Kb will be =
![[HC_(2)H_(2)O_(2)] [OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/zy3v5f9qw2lzagjr9833eaum2ggcc1fnph.png) /
/
![[C_(2)H_(3)O_(2)^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/7ag6ibe2ph5nxvq241dk0r0juzkrntefne.png)
= Kw / Ka
= 1 x
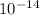 / 1.8 x
/ 1.8 x
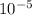 = 5.6 ×
= 5.6 ×
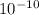
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png) = 2.4 ×
= 2.4 ×
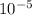
Now, we know , pOH = -log
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png) = 4.62
= 4.62
So, the pH = 14 - pOH = 14 - 4.62 = 9.38