First, we need to find out how many moles we have of
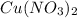
if we are given 6.35g.
We need to calculate the molar mass of the molecule:
Cu: 63.546g
N: 14.01g * 2 = 28.02g
O: 15.999g * 6 = 95.994
Add them together to get: 187.56g
So now we need to find the number of moles we are given. This can be done by setting up a multiplication equation and cancelling the units:
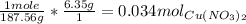
So now that we know the number of moles that we have, we can use the molarity equation to find the volume of the solution. The molarity equation is:
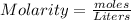
So since we are given the molarity in the equation (0.750 M), we can plug in the molarity and moles to find the volume of the solution:
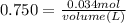
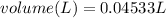
So now we know that the volume is 0.04533 Liters; however the question asks for the answer in milliliters. To do this, we must multiply by 1000:
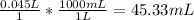
So
the volume of the solution (in mL) is 45.33 mL.