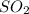Answer: There will be 131.3 tons of
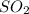 that will be generated.
that will be generated.
Explanation : We have the data as 72 tons of
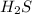 ,
,
 is 101 tons and
is 101 tons and
 as 38 tons.
as 38 tons.
Conversion of 1 ton to kg will be approximately 907.2 Kg
We have to convert the given data of tons into kg for all;
So, moles of
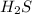 = (72 tons X 907.2 Kg/ton X 1000 g/Kg) / (34.082 g/ mol) = 1.916 X
= (72 tons X 907.2 Kg/ton X 1000 g/Kg) / (34.082 g/ mol) = 1.916 X
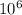 moles
moles
Now, moles of
 = (101 tons X 907.2 Kg/ton X 1000 g/Kg) / (32 g/mol) = 2.80 X
= (101 tons X 907.2 Kg/ton X 1000 g/Kg) / (32 g/mol) = 2.80 X
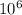 moles
moles
And,
 = (38 tons X 907.2 Kg/ton X 1000 g/Kg) / (18.016 g/mol)
= (38 tons X 907.2 Kg/ton X 1000 g/Kg) / (18.016 g/mol)
= 1.03 X
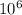 moles
moles
Now, we have to calculate the moles of
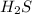
so, 1.916 X
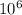 moles / 2 moles of
moles / 2 moles of
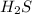 = 958 X
= 958 X
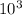
Now, for
 ;
;
So, 2.80 X
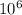 moles / 3 moles of
moles / 3 moles of
 = 933.3 X
= 933.3 X
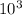 moles
moles
Here, we can see that
 is acting as a limiting reactant.
is acting as a limiting reactant.
Now, moles of
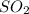 = 2.80 X
= 2.80 X
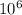 X (2 moles of
X (2 moles of
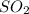 / 3 moles of
/ 3 moles of
 ) = 1.86 X
) = 1.86 X
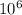
Now converting this into mass,
Mass = Moles X Molar Mass
Mass = 1.86 X
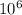 X (64.066 g/mol X
X (64.066 g/mol X
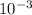 kg/g) X (1 ton / 907.2 Kg)
kg/g) X (1 ton / 907.2 Kg)
= 131.3 tons of