Answer:
The copper is solvent and tin is solute.
Step-by-step explanation:
Solution = Solute + solvent
Solute is that substance in a solution which gets dissolves in a solvent. it is always present in a lessor amount than the solvent.
Where as solvent is that part of solution in which solute gets dissolved.It is always present in a larger amount than that of the solute.
Mass of the sample = 130.0 g
Percentage of copper by mass = 90.0%
Mass of copper =
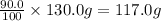
Percentage of tin by mass = 10.0%
Mass of tin=
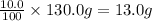
Since mass of copper is larger than that of tin which makes copper a solvent and tin a solute.