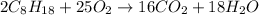Step-by-step explanation:
A balanced reaction is a reaction where there are equal number of atoms on both reactant and product side.
For example,
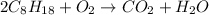
Number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
Therefore, in order to balance the equation we multiply
 by 25 on reactant side and multiply
by 25 on reactant side and multiply
 by 16 and
by 16 and
 by 18.
by 18.
Thus, the balanced chemical equation will be as follows.