Hi there!
• Avogadro's number = 6.023 × 10²³
• No.of molecules in N = 1.806 × 10²² [ Given ]
It's known that :-
Number of molecules = Moles × Avogadro's number
=> 1.806 × 10²² = Mol. × 6.023 × 10²³
=> Mole =
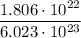
=> Moles = 0.03 mol.
Hence, 0.03 mol. is th' required answer.
~ Hope it helps!