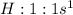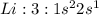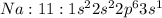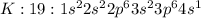Answer: C. hydrogen
Explanation:
Atomic radius of an atom is defined as the total distance from the nucleus to the outermost orbital of electron.
On moving from top to bottom, there is an addition of shell around the nucleus and the outermost orbital gets far away from the nucleus and hence, the distance between the nucleus and outermost orbital increases.
This in turn increases the atomic radii of the element from moving top to bottom in a group.