Answer:
Quantities of CCl₄ = 153. 8227 g/mol
Step-by-step explanation:
Thinking process:
Data:
The molar mass of CCl₄ is determined by adding the relative atomic masses in the compound. In this case:
Molar mass of chlorine, Cl = 35.453 amu (atomic mass units) or 35.453 g/mol
Molar mass of carbon, C = 12.0107 amu (lamu (atomic mass units) or 12.0107g/mol
Remember, 1 mole or carbon contains 12.0107 amu
The compouns contains: 1 carbion atom= 12.0107g/mol
4 cholrine atoms = 4 * 35.453 g/mol
= 141. 812 g/mol
Adding the atomic masses to get the total molecular mass of CCl₄ = 12.0107 + 4 (35.453) = 153. 8227 g/mol
Butn number of moles in 150.0g CCl4 =
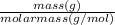
=
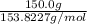
= 0.975 moles
Therefore, 150.0g CCl4 makes 0.975 moles
The student used 153. 8227 g/mol CCl₄ to make the conversion