Radioactive material undergoes first order dissociation kinetics.
For 1st order system,
k = 0.693 / t1/2
where, t 1/2 = half-life of the radioactive disintegration process.
Given that, t 1/2 =
73.83 days
Therefore, k = 0.009386 day-1
Also, for 1st order reaction,
k =
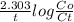
Given that, Co = initial concentration of Iridium-192 = 100 g
Therefore, 0.009386 =
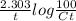
On rearranging we get, Ct = 100
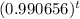
Answer: Ct = 100
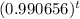 equation approximates the amount of Iridium-192 present after t days
equation approximates the amount of Iridium-192 present after t days