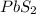Answer: The chemical formula for the compound formed will be
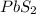
Step-by-step explanation:
Lead is the 82th element of the periodic table having electronic configuration of
![[Xe]4f^(14)5d^(10)6s^26p^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/6fwba5g0q2uxq25dk289lyt72xnnoi6qql.png)
This element has an oxidation state of +4 units.
Sulfur is the 16th element of the periodic table having electronic configuration of
![[Ne]3s^23p^4](https://img.qammunity.org/2019/formulas/chemistry/high-school/3thinf55xy4tqgy9frdwyiyh0nvf6yfhu8.png)
This element requires 2 electrons and thus shows an oxidation state of -2 units.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
Thus, the chemical formula for the compound formed will be