There's three types of decay: alpha beta and gamma.
*
Alpha decay is the emission of a helium nucleus (2protons and 2 neutrons)
*
Beta decay is the emission of an electron or a positron (
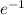
or
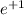
). It does affect the weight of the atom.
*
Gamma decay is the emission of photons with a high energy. It does affect the weight of the atom.
To answer the question,
any nuclear reaction which decreases the atomic weight by 4 and the protons by 2 is an alpha decay. (release of an alpha or helium nucleus)