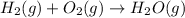Answer :
Combustion reaction : The combustion means burning. It is defined as the reaction in which a hydrocarbon react with oxygen in the presence of air to give carbon dioxide and water vapor as a product.
The combustion of methane gives carbon dioxide and water vapor as a product but the combustion of hydrogen gives water vapor as a product.
The balanced combustion reaction of methane will be:
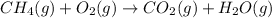
The balanced combustion reaction of hydrogen will be: