The question is incomplete. Complete question is read as:
'
One cup of fresh orange juice contains 115 mg of ascorbic acid (vitamin C, C6H8O6). Given that one cup = 218.0 mL calculate the molarity of vitamin C in organic juice.'
..........................................................................................................................
Answer:
Given: weight of solute (ascorbic acid) = 115 mg = 0.115 g
Volume of solution = 218.0 mL = 0.218 L
Molecular weight of ascorbic acid = 176.12 g/mol.
Now, Molarity =

=
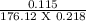
= 0.002995 mol/dm3
Answer: Molarity of solution = 0.002995 mol/dm3