Answer:
1.25 g of radioactive isotope will remain in the sample after 3 half-lives.
Step-by-step explanation:
Formula used to calculate the number of half life elapsed :
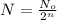
where,
N = amount of radioactive sample remains after n-half lives = ?
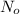 = Initial amount of the radioactive sample= 10 g
= Initial amount of the radioactive sample= 10 g
n = number of half lives = 3
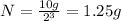
1.25 g of radioactive isotope will remain in the sample after 3 half-lives.