we can use the combined gas law equation as the amount of gas is constant in both instances
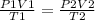
P - pressure , V- volume and T - temperature in kelvin
parameters for the first instance are on the left side and parameters at STP are on the right side of the equation
P1 - 97 000 Pa
P2 - 101 325 Pa
T1 - 35.0 °C + 273 = 308 K
T2 - 273 K
V1 - 140 mL
V2
substituting the values in the equation
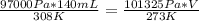
V = 119 mL
volume at STP is 119 mL