Molarity of solution is expressed as,
Molarity =
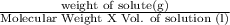
Molecular weight of KCl = 74.55 g/mol
For preparing 1 mol of KCl solution (1 liter), 74.44 g KCl must be dissolved in 1 liter of water.
Following glasswares are required for preparing the solution.
1) Watch Glass (for weight the KCl)
2) Beaker (for preparing the solution)
3) Stirrer (For dissolving KCl in water)
4) Standard measuring flask (To ensure final volume of solution is 1 liter)