Answer : The correct answer for molarity of MgCl₂ solution is 0.67 M.
Molarity :
It expresses concentration of any solution . It can be defined as mole of solute present in volume of solution in Liter .
It uses unit M or
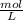
It can be formulated as :
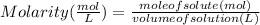
Given : mol of MgCl₂ (solute ) = 0.50 mol
Volume of solution = 750 mL
Following steps can be done :
Step 1 ) To convert volume of solution from mL to L
1 L = 1000 mL
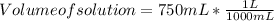
Volume of solution = 0.750 L
Step 2) To find molarity
Plugging mole of solute and volume of solution in molarity formula as :
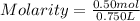
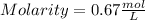
Hence molarity of MgCl₂ solution is 0.67 M