Answer:
Some thermal energy is lost and some is used to do work
Step-by-step explanation:
The first law of thermodynamics sates that the energy is neither created nor destroyed. This law basically states the conservation of energy.
Mathematically, it can be written as :
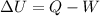
Where,
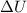 is the change in internal energy
is the change in internal energy
Q is the heat absorbed
W is the work done
Second law states that for an isolated system the entropy should increase or remains the same. It will never decrease.
The relationship between the first and the second law is (2) " Some thermal energy is lost and some is used to do work ".