Molarity is the most convenient way to express concentration.
Mathematically, molarity is expressed as
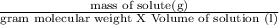
In present case,
mass of solute (KCl) = 36 g
molecular weight of KCl = 74.55 g/mol
volume of solution = 100 ml = 0.1 l
∴ Molarity =
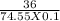
= 4.829 M
Thus, conc. of saturated KCl at 20 oC is 4.829 M