Answer:
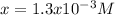
Step-by-step explanation:
Hello,
in this case, one considers the dissolution as an ionic reaction:
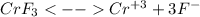
In such a way, we write the equilibrium equation based on the change
 due to the slight rate of dissolution of the considered salt as:
due to the slight rate of dissolution of the considered salt as:
![Ksp=[Cr^(+3) ]_(eq) [F^(-) ]_(eq) ^(3) \\Ksp=x(3x)^(3) \\x=\sqrt[4]{(Ksp)/(27)} =\sqrt[4]{(6.6x10^(-11) )/(27)}\\x=1.3x10^(-3)M](https://img.qammunity.org/2019/formulas/chemistry/college/g3zw0a76o26ka64iejx5gd1gou57k0jip9.png)
Such
 accounts for the molar solubility of the chromium (III) fluoride.
accounts for the molar solubility of the chromium (III) fluoride.
Best regards.