Answer: The heat of reaction (ΔHrxn) for the reaction is -164.9kJ
Step-by-step explanation:
The given balanced chemical reaction is,
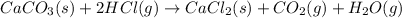
To calculate the enthalpy of reaction
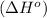 .
.
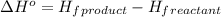
![\Delta H^o=[n_(CaCl_2)* \Delta H_f^0_((CaCl_2))+n_(CO_2)* \Delta H_f^0_((CO_2))+n_(H_2O)* \Delta H_f^0_((H_2O))]-[n_(CaCO_3)* \Delta H_f^0_{(CaCO_3)+n_(HCl)* \Delta H_f^0_((HCl))]](https://img.qammunity.org/2020/formulas/chemistry/college/836vrkb6d5a1dd92jh1m31699ezsxb967v.png)
where,

Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(1* -877.1)+(1* -393.51)+(1* -285.8)]-[(1* -1206.9)+(2* -92.30)]=-164.9kJ](https://img.qammunity.org/2020/formulas/chemistry/college/b1aho2fxeuqy9nxwhux788jw6z8beovuza.png)
Therefore the heat of reaction (ΔHrxn) for the reaction is -164.9kJ