Answer:
Volume of the container = 0.0618 m³
Step-by-step explanation:
Assuming that the gas is ideal, we can apply ideal gas equation:
PV=nRT
where P = pressure of the gas
V = volume occupied by the gas
n = number of moles of gas
R = Universal gas constant = 8.314 J/mol-K
T = Temperature of the gas
Here we have to find volume.
Given: P=2.25 atm T=385 K n=4.39 moles
Putting the above values in the ideal gas equation, we get:
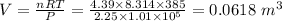
Volume of the gas = volume of the container in which it is filled = 0.0618 m³