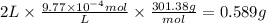Answer:
0.589 g
Step-by-step explanation:
The absorbance (A) of an analyte is related to its concentration (c) through the Beer-Lambert's law.
A = ε . l . c
where,
ε: molar absorptivity
l: optical path length
A = ε . l . c
0.687 = 703 M⁻¹.cm⁻¹ . 1 cm . c
c = 9.77 × 10⁻⁴ M
The molar mass is 301.38 g/mol and the volume of solution is 2 L. The mass of Dobutamine is: