Answer: The given statement is true.
Step-by-step explanation:
Average kinetic energy is defined as the mean of all the energies present within the molecules of a substance.
Also, we known that relation between kinetic energy and temperature is as follows.
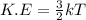
Since, kinetic energy is directly proportional to temperature. So, when there will be increase in temperature then this will also lead to increase in kinetic energy. Hence, average kinetic energy will also increase.
When we place two objects of different temperatures adjacent to each other then there will occur transfer of heat from hotter object to colder object till their temperature becomes equal.
Thus, we can conclude that the statement in general, heat flows from an object possessing molecules of higher average kinetic energy to an object possessing molecules with lower average kinetic energy, is true.