Answer:
Hi, the given question has some missing part. Complete question is: " For a chemical reaction to be non-spontaneous at any temperature, which of the following conditions must be met?
A. ΔS° > 0, ΔH° > 0
B. ΔS° > 0, ΔH° < 0
C. ΔS° < 0, ΔH° < 0
D. ΔS° < 0, ΔH° > 0
E. All reactions are spontaneous at some temperature."
Option (D) is correct.
Step-by-step explanation:
We know that,
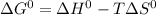
Where T represents temperature in kelvin scale. Hence it is always positive.
For a non-spontaneous reaction,
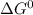 should be positive.
should be positive.
If
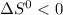 then
then
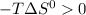 at any T value.
at any T value.
Hence
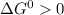 for any value of T if both
for any value of T if both
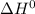 and
and
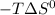 are greater than zero.
are greater than zero.
So, option (D) is correct.