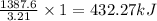Answer: The ΔH of the reaction if 51.3 g of
 reacts with excess
reacts with excess
 to yield 1387.6 kJ is 432.27kJ
to yield 1387.6 kJ is 432.27kJ
Step-by-step explanation:
To calculate the moles, we use the equation:
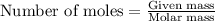
moles of

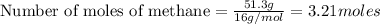
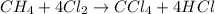
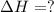
As
 is present in excess,
is present in excess,
 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.
If 3.21 moles of methane releases heat = 1387.6 kJ
Thus 1 mole of methane release=