Answer:
Work function of the metal is 3.24 eV.
Step-by-step explanation:
It is given that,
Wavelength,
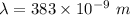
Let W is the work function of the metal. It is given by using Einstein's photoelectric effect equation. It is given by :
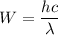
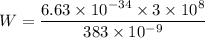
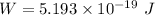
Since,
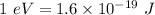
So, W = 3.24 eV
So, the work function of the metal is 3.24 eV. Hence, this is the required solution.