Answer:
85.5 %
Step-by-step explanation:
Before we can simply apply the equation needed for this problem, let's clarify several concepts and understand the meaning of that specific equation.
Theoretical yield is defined as the maximum mass that can be produced when some specific reaction takes place. Theoretical yield in this case is given by:
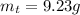
Actual amount of product would always be a smaller number, since in lab conditions we may lose some mass of the product due to various reasons, such as not being able to recrystallize it fully or just losing some fraction of it due to specific errors in experimental technique. In this case, the actual amount of the product recovered is:
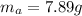
To find the percent yield of the reaction, we need to understand that the percent yield is simply a fraction of how much product was recovered relatively to the maximum amount of product that can be recovered. This fraction might be expressed in percentage if we multiply by 100 %:
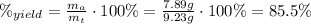
Have a great day as well!