Answer:
Therefore, the amount of heat produced by the reaction of 42.8 g S = (-5.2965 × 10²) kJ = (-5.2965 × 10⁵) J
Step-by-step explanation:
Given reaction: 2S + 3O₂ → 2 SO₃
Given: The enthalpy of reaction: ΔH = - 792 kJ
Given mass of S: w₂ = 42.8 g, Molar mass of S: m = 32 g/mol
In the given reaction, the number of moles of S reacting: n = 2
As, Number of moles:
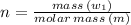
∴ mass of S in 2 moles of S:
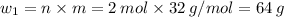
Given reaction: 2S + 3O₂ → 2 SO₃
In this reaction, the limiting reagent is S
⇒ 2 moles S produces (- 792 kJ) heat.
or, 64 g of S produces (- 792 kJ) heat.
∴ 42.8 g of S produces (x) amount of heat
⇒ The amount of heat produced by 42.8 g S:
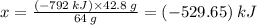
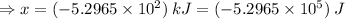
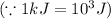
Therefore, the amount of heat produced by the reaction of 42.8 g S = (-5.2965 × 10²) kJ = (-5.2965 × 10⁵) J