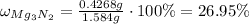Answer:
26.95 %
Step-by-step explanation:
Air contains the highest percentage of oxygen and nitrogen gases. Magnesium then combines with both of the gases:
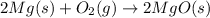
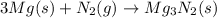
Firstly, find the total number of moles of magnesium metal:
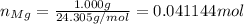
Let's say that x mol react in the first reaction and y mol react in the second reaction. This means:
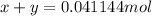
According to stoichiometry, we form:
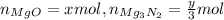
Multiplying moles by the molar mass of each substance will yield mass. This means we form a total of:
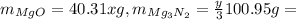
The total mass is given, so we have our second equation to solve:
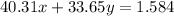
We have two unknowns and two equations, we may then solve:
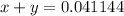
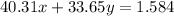
Express y from the first equation:
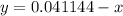
Substitute into the second equation:
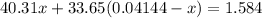
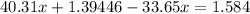
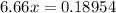
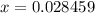

Moles of nitride formed:
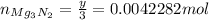
Convert this to mass:
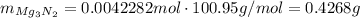
Find the percentage: