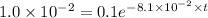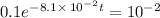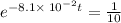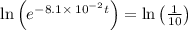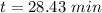Answer:
28.43 min
Step-by-step explanation:
Using integrated rate law for first order kinetics as:
![[A_t]=[A_0]e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/college/wgh5hifj7f12vitsa51kophgqrxxcfit2c.png)
Where,
![[A_t]](https://img.qammunity.org/2020/formulas/chemistry/college/wbj92t0z4axifcyqa24z3ary269op2iva8.png) is the concentration at time t
is the concentration at time t
![[A_0]](https://img.qammunity.org/2020/formulas/chemistry/college/izynxfnwyud2ghdog9l8ny0mhzwshbud6r.png) is the initial concentration
is the initial concentration
Given that:
The rate constant, k =
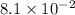 min⁻¹
min⁻¹
Initial concentration
![[A_0]](https://img.qammunity.org/2020/formulas/chemistry/college/izynxfnwyud2ghdog9l8ny0mhzwshbud6r.png) = 0.1 M
= 0.1 M
Final concentration
![[A_t]](https://img.qammunity.org/2020/formulas/chemistry/college/wbj92t0z4axifcyqa24z3ary269op2iva8.png) =
=
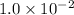 M
M
Time = ?
Applying in the above equation, we get that:-