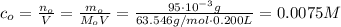Answer:
0.0075 M
Step-by-step explanation:
Mass of copper metal recovered:
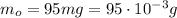
Molar mass of copper should be used in this problem in order to convert the mass of copper into moles of copper:
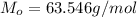
According to the balanced chemical equation, 1 mole of copper(II) sulfate produces 1 mole of copper metal, this means, when we find the number of moles of copper metal, this will be equivalent to the number of moles of copper(II) sulfate. Dividing the number of moles of copper(II) sulfate by the volume of the solution in liters will yield the molarity needed.
Firstly, the number of moles of copper (and copper(II) sulfate):
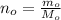
Given a volume of:
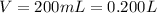
We obtain a molarity of: